A new scientific discipline
2025 has been designated the International Year of Quantum Science and Technology by UNESCO, as it was 100 years in September since Heisenberg published his seminal paper, “Über quantentheoretische Umdeutung kinematischer und mechanischer Beziehungen” (On the quantum-theoretical reinterpretation of kinematical and mechanical relationships) in Zeitschrift für Physik.1 Although Heisenberg surely didn’t realise it at the time, this paper heralded a brand new scientific discipline that would completely change physical and chemical science, and it is very appropriate that events celebrating the birth of quantum mechanics are being held around the globe this year – it’s a pretty big deal!
2025 also marks a much lesser-known anniversary, albeit one that should be of interest to many chemists. In 1925, two papers by Walter Noddack, Ida Tacke, and Otto Berg appeared in the literature; the first, in June, titled, “Die Ekamangane” (The eka-manganeses) was published in Die Naturwissenschaften,2 and the second, “Zwei neue Elemente der Mangangruppe” (Two new elements of the manganese group) was published in Sitzungsberichte der Preussischen Akademie der Wissenschaften.3 In these papers, the authors reported the discovery of two new elements, number 43, which they named masurium, and 75, which they named rhenium. While controversy still rages over the discovery of the former (Noddack, Tacke and Berg’s claim was rejected, element 43 is now named technetium and its official discovery date is 19374), there is no doubt about the latter. And what makes rhenium especially interesting and worth celebrating in 2025 is that it was the last stable ‘naturally occurring’ element to be discovered. But what is meant by this?
It might be argued that any element that exists for any finite amount of time on planet Earth, and which doesn’t require human intervention for its synthesis, could be classified as being naturally occurring. However, a stable naturally occurring element is one that has at least one non-radioactive isotope, and which has therefore existed on Earth from its formation until the present day. Thus technetium (Z = 43) and promethium (Z = 61), as well as the elements from bismuth (Z = 83) to plutonium (Z = 94), while naturally occurring (although in truly miniscule amounts in some cases), are not stable, because all their isotopes are radioactive. And this then brings us back to rhenium.
Mendeleev’s original periodic table looked very different to that of today. The genius behind his discovery was that he knew that not all elements had been discovered in 1869, the year in which he proposed the periodic table, and he therefore left gaps that would be filled in the future. These gaps were indeed duly filled, and by the start of the 20th century, the only gaps remaining amongst the first 82 elements were those at Z = 43, 61, 71, 72 and 75. Elements 43 and 61, as we have seen above, are the unstable technetium and promethium, respectively. Lutetium (Z = 71) was discovered in 1907 and hafnium (Z = 72) in 1923, leaving element 75 as the last stable naturally occurring element yet to be discovered. Or was it?
In 1908, having obviously been conscious of the conspicuous gaps in the periodic table beneath manganese, the Japanese chemist Masataka Ogawa reported the discovery of element 43, and named it nipponium (Np).5 He had been working in the lab of Sir William Ramsay at University College, London from 1904-1906, studying a sample of the mineral thorianite (ThO2), and analysis of extracts of this gave results consistent with the presence of a new element. On his return to Japan he improved his extraction process and isolated around 100 mg of what was thought to be an oxide of nipponium. This new element exhibited spectral lines that did not match those of any other, and Ogawa proposed it to be element 43 with a molar mass of around 100, this being based on the assumption of a 2+ oxidation state for the new element. It is now generally believed that Ogawa’s nipponium was in fact rhenium, rather than what would have been technetium. Analysis of his data using modern techniques has given results consistent with compounds of rhenium being obtained from the extraction process, and it does now appear that Ogawa has a significant claim to be the discoverer of rhenium.6,7
However, most references give priority for rhenium to Noddack, Tacke, and Berg, citing their two abovementioned papers from 1925. These workers studied platinum ores and the mineral columbite (Fe,Mn)Nb2O6, extracting small amounts of compounds containing what they claimed were both elements 43 and 75. X-ray spectra of these were proposed to be consistent with two new elements, and they were duly named masurium, from Masuria in East Prussia (now Poland), and rhenium, after the Rhine river. It is now thought unlikely that the X-ray apparatus used by Noddack, Tacke and Berg would have been sensitive enough to detect the minute amounts of technetium present in some ores, and priority for the discovery of this element is now given to Segré and Perrier (1937).4
Interest in rhenium was modest in the first 30 or so years following its discovery, with fewer than 100 papers appearing per year every year until 1957. The 1960s saw a significant increase in the number of papers published, and following a plateau in the 1970s and 80s, the number of papers concerning this element has steadily increased to the point where nearly 2000 papers per annum are now published.
The upsurge of interest in rhenium in the 1960s was undoubtedly a product of the syntheses and characterisation of some truly unprecedented compounds. The first metal-metal double, triple and quadruple bonds were found in rhenium compounds, and Kiwi chemists were at the forefront of two of these discoveries. The known dark-red compound having the empirical formula CsReCl4 was first shown, in a rather innocuous-looking ¾ page paper published in 1963, to have the unprecedented structure depicted in Fig. 1, with the chemical formula Cs3Re3Cl12, and in which the rhenium ions are doubly bonded to each other.8 The names of the authors of this paper, Cantabrians Ward Robinson, Jack Fergusson, and Bruce Penfold, will be well known to the (older) New Zealand chemistry community, as possibly will be the controversy involving F. A. Cotton over priority for the discovery.
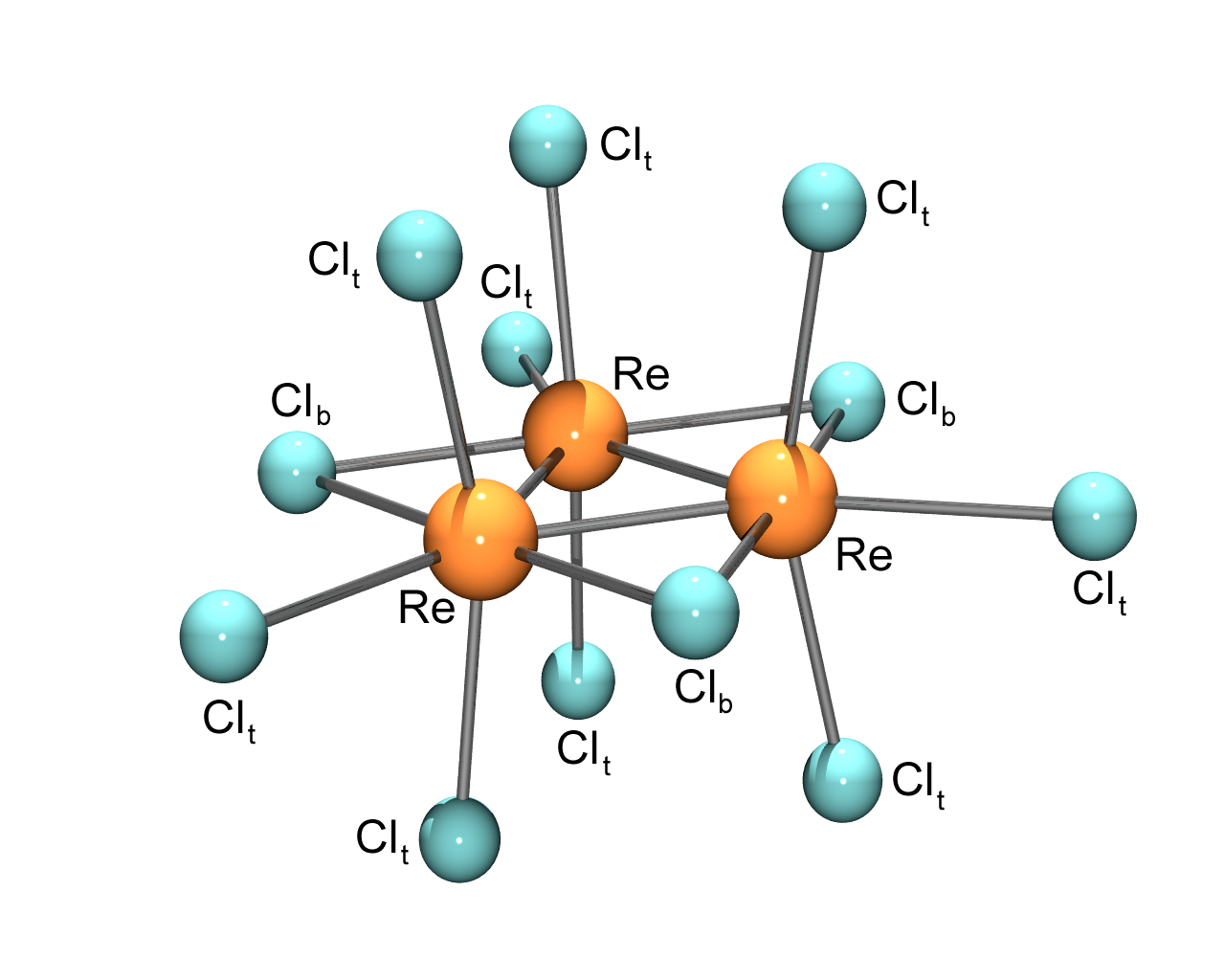
Fig. 1. Structure of the Re3Cl123- complex anion. The t and b subscripts refer to terminal and bridging chlorido ligands, respectively.
Around the same time, Neil Curtis (Victoria University), while a research associate at MIT, first made a compound with the same CsReCl4 empirical formula as that above, except his was royal blue.9 The potassium salt of this was later shown to have the chemical formula K2Re2Cl8, and the utterly remarkable structure shown in Fig. 2, in which the Re ions are connected by a quadruple bond.10 In fact, Russian workers were the first to obtain a crystal structure of the quadruply bonded Re2Cl82- ion, as the bis(pyridinium) salt in 1963, with the Re-Re bond distance being 2.22 Å.11

Fig. 2. Structure of the Re2Cl82- complex anion
Another remarkable rhenium compound from the 1960s was the ReH92- complex anion, which was first characterised as its dipotassium salt by both X-ray and neutron diffraction in 1964.12,13 This, and its technetium analogue, still appear to be the only examples of complexes in which a transition metal ion is surrounded by 9 monodentate ligands (Fig. 3).
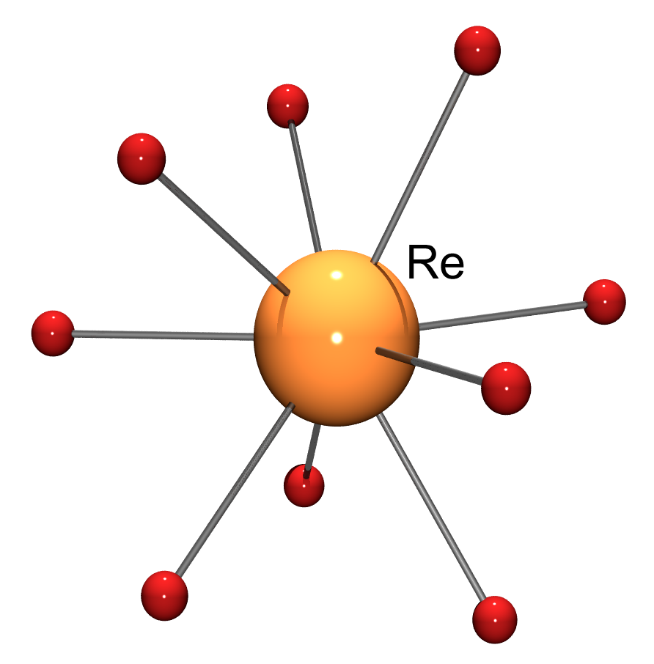
Fig. 3. Structure of the ReH92- complex anion. A tricapped trigonal prismatic geometry obtains about the Re(VII) ion.
The organometallic chemistry of rhenium also developed extensively in the 1950s and 1960s, building on the earlier synthesis of the singly metal-metal bonded Re2(CO)10 in 1941.14 Of particular interest during this time was the complex [Re(C5H5)2H], the first transition metal hydride unsupported by carbonyl ligands. This was reported in 1955 as the product of the reaction of ReCl5 and NaC5H5, and was a very early example of a transition metal complex being characterised by 1H NMR, the hydride resonance being found 17.2 ppm upfield of the water signal.15 Surprisingly, it appears that the first structural characterisation of this complex has been carried out only recently (2022, Fig. 4).16

Fig. 4. Structure of [Re(C5H5)2H]. The hydride H atom was not located in the X-ray structure.
So what became of the major protagonists in the rhenium story? Ogawa died in 1930, weeks after analysis of his sample of ‘nipponium’ using a new X-ray spectrometer at The Imperial University of Tokyo showed it ‘was beautiful rhenium indeed’.7 Noddack and Tacke married in 1926, both subsequently publishing under the former surname, and they were awarded the Liebig Medal of the Gesellschaft Deutscher Chemiker in 1931 for their achievements. However, they did not withdraw their claim to the discovery of element 43 even after the authentic element was synthesised, and this was said to be ‘a stain on their reputation’.17 In 1934, Ida was one of the first to propose that the atomic nucleus could split on bombardment with neutrons, in a critique of Fermi’s claim to have obtained element 93 by neutron irradiation of uranium.18 During World War II, the couple moved to The University of Strasbourg, but after the war they had difficulty finding academic positions in Germany; Walter eventually obtained an appointment at The Bamberg University of Philosophy and Theology, with Ida as his unpaid assistant. Walter died in 1960 and Ida in 1978. Sadly, Otto Berg being of Jewish descent, was forced to leave Germany for England in 1938. He died there in 1939.
And what of the element rhenium in its centenary year? Global annual production of rhenium is around 40-50 tonnes, the majority of which comes from Chile, and the current price is approximately USD $4,000 per kilogram. As alluded to above, interest in the chemistry of rhenium, 100 years on from its discovery, is steadily increasing and a relatively recent review should satiate the curious reader.19
References
- Heisenberg, W. Über Quantentheoretische Umdeutung Kinematischer Und Mechanischer Beziehungen. Z. Für Phys. 1925, 33 (1), 879–893. https://doi.org/10.1007/BF01328377.
- Noddack, W.; Tacke, I. Eka-Manganeses. I. Chemical Part. Naturwissenschaften 1925, 13, 567–571. Berg, O.; Tacke, I. Eka-Manganeses. II. X-Ray Spectroscopic Part. Naturwissenschaften 1925, 13, 571–574.
- Noddack, W.; Tacke, I. Two New Elements of the Manganese Group. I. Chemistry. Sitzb Preuss Akad Wiss. 1925, 400-405. Berg, O.; Tacke, I. Two New Elements of the Manganese Group. II. X-Ray Spectroscopic Part. Sitzb Preuss Akad Wiss. 1925, 405-409.
- Perrier, C.; Segre, E. Radioactive Isotopes of Element 43. Nature. 1937, 140, 193-194. https://doi.org/10.1038/140193b0.
- Ogawa, M. Preliminary Note on a New Element (Nipponium) in Thorianite. J. Coll. Sci. Imp. Univ. Tokyo 1908, 25, 1-11.
- Yoshihara, H. K. Nipponium as a New Element (Z=75) Separated by the Japanese Chemist, Masataka Ogawa: A Scientific and Science Historical Re-Evaluation. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2008, 84 (7), 232–245. https://doi.org/10.2183/pjab.84.232.
- Hisamatsu, Y.; Egashira, K.; Maeno, Y. Ogawa’s Nipponium and Its Re-Assignment to Rhenium. Found. Chem. 2022, 24 (1), 15–57. https://doi.org/10.1007/s10698-021-09410-x.
- Robinson, W. T.; Fergusson, J. E.; Penfold, B. R. The Configuration of the Anion in CsReCl4. Proc. Chem. Soc. Lond. 1963, 116.
- Multiple Bonds Between Metal Atoms, Third Edition; Cotton, F. A., Murillo, C. A., Walton, R. A., Eds.; Springer, 2005. https://doi.org/10.1007/b136230.
- Cotton, F. A.; Harris, C. B. The Crystal and Molecular Structure of Dipotassium Octachlorodirhenate(III) Dihydrate, K2[Re2Cl8].2H2O. Inorg. Chem. 1965, 4, 330-333. https://doi.org/10.1021/ic50025a015.
- Kuznetsov, V. G.; Koz’min, P. A. The Structure of (pyH)HReCl4. Zhurnal Strukt. Khimii 1963, 4, 55–62.
- Knox, K.; Ginsberg, A. P. X-Ray Determination of the Crystal Structure of Potassium Rhenium Hydride. Inorg. Chem. 1964, 3 (4), 555–558. https://doi.org/10.1021/ic50014a025.
- Abrahams, S. C.; Ginsberg, A. P.; Knox, K. Transition Metal-Hydrogen Compounds. II. The Crystal and Molecular Structure of Potassium Rhenium Hydride, K2ReH9. Inorg. Chem. 1964, 3 (4), 558–567. https://doi.org/10.1021/ic50014a026.
- Hieber, W.; Fuchs, H. Über Metallcarbonyle. XXXVIII. Über Rheniumpentacarbonyl. Z. Für Anorg. Allg. Chem. 1941, 248 (3), 256–268. https://doi.org/10.1002/zaac.19412480304.
- Wilkinson, G.; Birmingham, J. M. Biscyclopentadienylrhenium Hydride-a New Type of Hydride. J. Am. Chem. Soc. 1955, 77, 3421. https://doi.org/10.1021/ja01617a098.
- Magott, M.; Brzozowska, M.; Baran, S.; Vieru, V.; Pinkowicz, D. An Intermetallic Molecular Nanomagnet with the Lanthanide Coordinated Only by Transition Metals. Nat. Commun. 2022, 13 (1), 2014. https://doi.org/10.1038/s41467-022-29624-7.
- Brazil, R. Ida Noddack and the trouble with element 43. Chemistry World. https://www.chemistryworld.com/culture/ida-noddack-and-the-trouble-with-element-43/4013548.article (accessed 2025-07-31).
- Noddack, I. Element 93. Angew. Chem. 1934, 47, 653-655. https://doi.org/10.1002/ange.19340473707.
- Dilworth, J. R. Rhenium Chemistry – Then and Now. Coord. Chem. Rev. 2021, 436, 213822. https://doi.org/10.1016/j.ccr.2021.213822.