Article first published in Chemistry in New Zealand Vol .76, No. 4. Oct. 2012, 129-132
Introduction
Cyanide uses and toxicity
Cyanide is extensively employed in a variety of industrial processes including the extraction of gold and silver from ores, electroplating baths, insecticides, case hardening of steels, analytical chemistry and the manufacture of other cyanides.1
Many cyanides are extremely toxic to terrestrial vertebrates and aquatic life. The cyanide anion is an inhibitor of the enzyme cytochrome c oxidase (a trans-membrane protein complex found in mitochondria and bacteria) attaching to the iron within this protein preventing the transport of electrons from the enzyme to oxygen. Consequently the eukaryotic cell can no longer aerobically produce ATP for energy and cellular respiration is greatly reduced affecting those tissues most dependant on it, especially the central nervous system and the heart.
Following oral administration, soluble cyanide salts at the pH encountered in the stomach form predominantly HCN which rapidly penetrates mucous and cell membranes. Acute exposure can result in nausea, anxiety, confusion, vertigo, dizziness, lower jaw stiffness, convulsions, spasms, paralysis, coma, irregular heartbeat, cyanosed (blue-grey coloured) skin, increased rate of breathing and death. The oral LD50 (rats) for potassium cyanide is 5 mg/kg body weight (bw), the oral LDLo (lowest dose known to have resulted in fatality) for humans is 2.8 mg/kg bw (or approximately 1 mg/kg bw as HCN).
The primary means the body employs to detoxify cyanide is its conversion to thiocyanate mediated by the enzyme rhodanese.2
Cyanide as a plant defence mechanism – cyanogenic glycosides
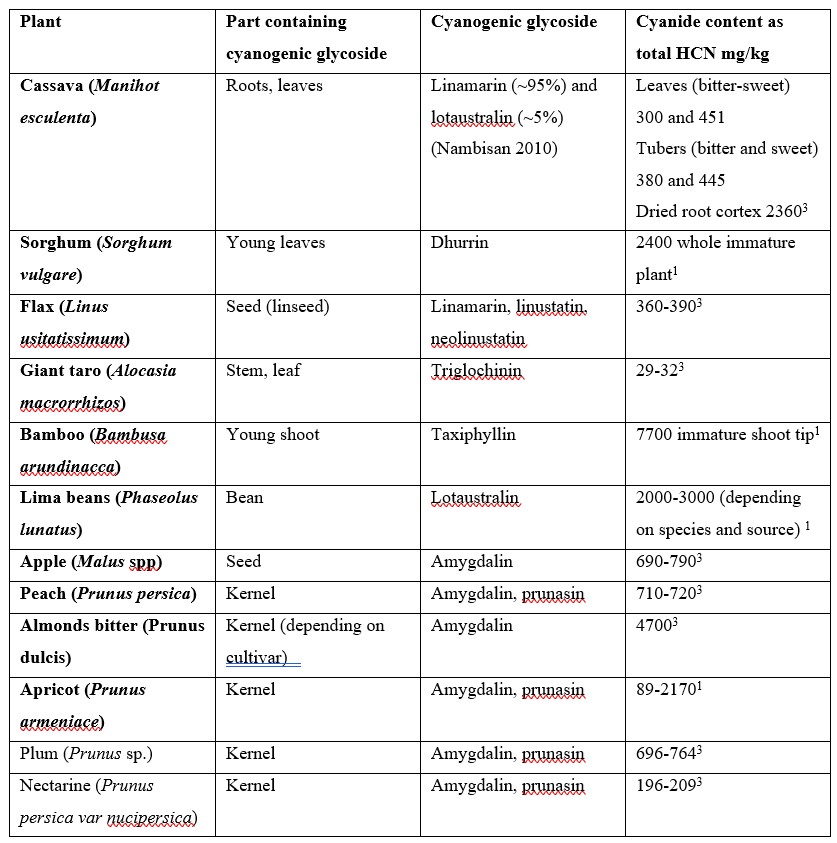
The use of cyanide as a poison by plants to deter predators precedes all human uses. Cyanide in plants appears mostly in the form of cyanogenic glycosides (CNG), i.e. a sugar attached to a cyanide group. At least 2,650 species of plants produce CNGs. The corresponding hydrolytic enzymes which are brought together when the cell structure of the plant is disrupted by a predator results in the rapid breakdown of the cyanogenic glycoside to a sugar and a cyanohydrin that rapidly decomposes to form HCN.3 There are approximately 25 CNGs known, some of which are found in major food crops and other edible plants including cassava, sorghum, lima beans, bamboo shoots, stone fruits and almonds. Cassava (also known as manioc, yuca and tapioca) is by far the most significant CNG containing food crop for humans, being the most important food in the tropics after rice and maize.4
Table 1. Cyanogenic glycosides in different foodstuffs.
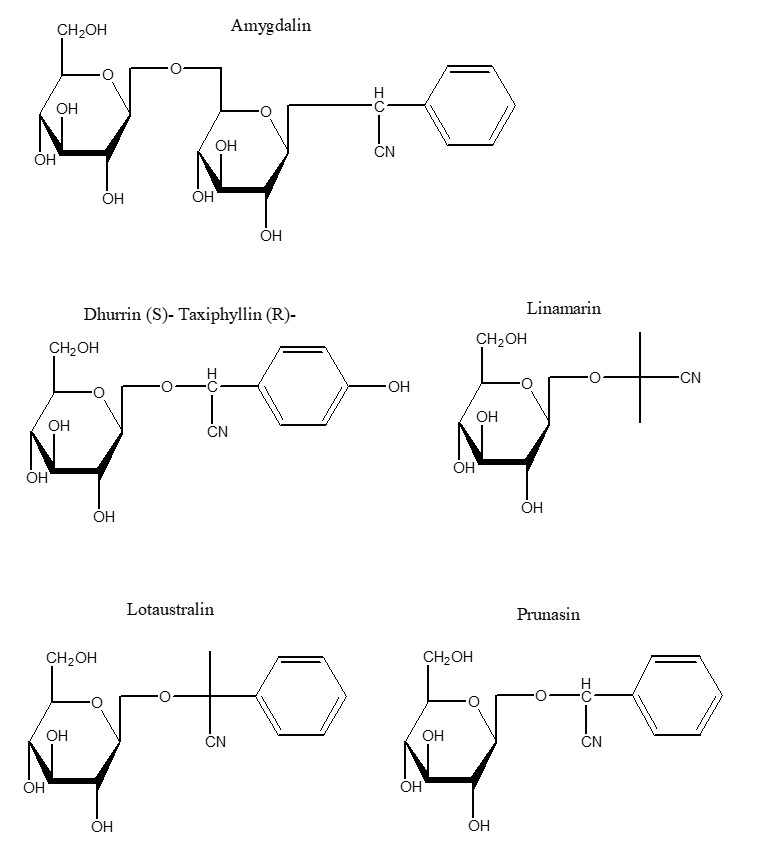
Effect of chronic exposure to cyanogenic glycoside containing foodstuffs
Liberation of HCN from CNGs usually occurs after mastication and ingestion and arises from enzymatic degradation of the latter to produce HCN resulting in acute or chronic cyanide poisoning. The enzyme responsible (β-glucosidase) may arise from the plant material or from gut microflora. HCN can also be produced to a lesser extent by glucosidases in the liver.5
While cyanide poisoning from CNGs most commonly occurs following ingestion, hydrolysis may also occur during food preparation. One graphic example involved a series of failed rescue attempts resulting in the poisoning of eight individuals in total exposed to HCN gas produced by bamboo shoots left to pickle in a well in Thailand (the cyanide content of the bamboo ranged from 39 to 434 mg/kg and the well volume was 27 m3). All victims lost consciousness, two died following cardiac arrest, four recovered with supportive care and two regained consciousness soon after the event.6
Chronic exposure to food containing CNGs has been linked to several diseases primarily affecting the central nervous system.
Tropical ataxic neuropathy (TAN) describes several neurological symptoms affecting the mouth, eyesight, hearing and gait that represent clinically as sore tongue, optical atrophy, angular stomatitis (inflammatory lesions at the corners of the mouth), neurosensory deafness, skin desquamations (peeling or shedding of outer skin layers) and sensory gait ataxia (deviation from normal walking). It occurs only rarely in children under 10 years of age and the diet of patients is usually comprised of a monotonous intake of cassava derivatives.
Spastic paraparesis (called mantakassa in Mozambique and konzo in the Democratic Republic of Congo) is a motor neuron disease characterised by weakness or paralysis of the legs. It often has an abrupt onset and most frequently affects children and women of child bearing age. The condition can sometimes affects the arms and speech. Epidemics of konzo have all been associated with chronic intake of cyanogenic glycosides at sub-lethal concentrations from cassava or cassava flour in combination with a low intake of sulfur-containing amino acids.
Goitre and cretinism due to iodine deficiency can be exacerbated by chronic consumption of inadequately processed cassava. Cyanogenic glycosides are converted to thiocyanate during the detoxification process, this reacts with iodine which is subsequently unavailable to the thyroid increasing the dietary requirement for iodine.7
Methods of reducing or eliminating toxicity
While genetic manipulation of CNG metabolism or selective breeding of cultivars offer a potential means of controlling the formation of CNGs and their distribution in plants, processing to remove CNGs remains the most practical method of reducing the levels of these toxic compounds in foodstuffs.
A plethora of different techniques are employed by communities for whom cassava represents a substantial portion of their diet including peeling and slicing followed by boiling, baking, steaming, drying, deep frying, fermentation, grating/pounding followed by drying and roasting. These processes differ in their effectiveness but remove CNG either by enzymatic hydrolysis with the subsequent loss of the volatile HCN produced or by being leached out with water. Cutting cassava into small pieces and boiling in water can remove up to 80% of CNGs that are subsequently leached into the water which is decanted repeatedly. The removal of CNG during drying is affected by the enzyme linamarase which can act on the cassava for a protracted period. In contrast, baking, steaming and frying are the least effective means of removing CNG (approximately 20% removed) due to the inactivation of linamarase and the stability of the CNG linamarin. The most effective means of removal are crushing and sundrying (< 5% retained) and grating/fermentation, dewatering and drying (< 2% retained).8
Total cyanide in cassava: analytical method
The analysis of CNGs generally involves three steps: (i) extraction from foodstuff, (ii) hydrolysis of cyanide and (iii) analysis of cyanide. Extraction is normally carried out in dilute acid followed, in the case of cassava, by enzymatic hydrolysis using linamarase. The enzyme, however, is expensive while acid digestion is cheaper can hydrolyse all of the CNGs. Acid hydrolysis based primarily upon the method of Haque & Bradbury (2002)3 was chosen because being non-specific the technique can accommodate a greater diversity of foodstuffs than autolysis or enzymatic hydrolysis.
Preparation of standards
Standards were prepared fresh for each analytical run from KCN. Approximately 12 mg was weighed accurately into a 5 ml volumetric flask and made up to volume with 0.1M H3PO4. A 100 mg/L as HCN working standard was prepared from this solution using a conversion factor KCN to equivalent HCN of X 0.415. The working solution was used to prepare standards in the following concentrations: 0.5, 1.0, 2.5, 5.0, 10.0 and 20.0 mg/L as HCN in 0.1M H3PO4. These solutions were treated as for sample extracts but without being heated in a water bath and equate to approximately 5, 10, 25, 50, 100 and 200 mg/kg cyanide as HCN in the original sample prior to extraction/dilution.
Extraction
Samples were homogenised in a blender (raw foodstuffs were frozen or assayed immediately to avoid breakdown of CNGs due to endogenous enzymes) and approximately 10 g accurately weighed into a 100 ml Schott bottle. 100 ml of 0.1M H3PO4 was added, the contents were shaken for 2 minutes and then left overnight at 5°C. The resultant solution was then centrifuged at 3000 rpm in a 50 ml centrifuge tube.
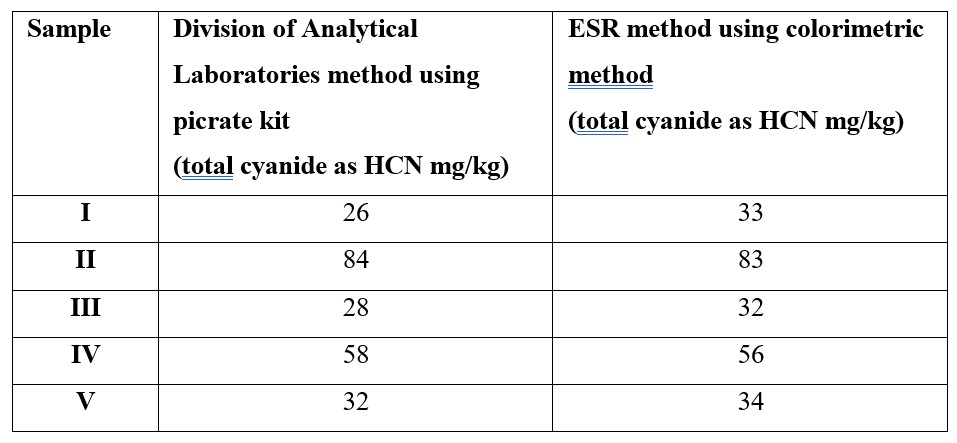
The method of Haque and Bradbury3 does not give an extraction time for the initial extraction into 0.1M H3PO4. It was found on comparison between the ESR determination and that performed by an Australian laboratory on the same samples (see Table 2) that samples left to extract overnight gave comparable results.
Digestion
1.6 ml portions of the interstitial layer were pipetted into multiple 8 ml capacity, sealable glass vials labelled respectively 0, 20, 40, 60, 80, 120, 180, 240, 300 and 360 minutes corresponding to the length of time the vial thus labelled was to remain in the water bath. 1.6 ml of 4M H2SO4 was added and the vials immediately sealed. The vials were then placed in a water bath heated to 100°C. Upon removal (or in the case of time = 0 minutes, immediately) the vials were placed in an ice bath and 4 ml of 3.6M NaOH added to halt the digestion process and convert cyanide to the stable NaCN.
Linamarin requires approximately 60 minutes under the conditions described above for complete hydrolysis but while undergoing hydrolysis the vial is losing HCN gas at a slow rate. The rate of loss is determined by graphing equivalent HCN concentration in each digest against time spent digesting and extrapolating back to T = 0.
Upon standing following the addition of 3.6M NaOH it was noted that a brown coloured precipitate formed. The precipitant was removed from each solution by filtering through a 0.45µm syringe filter, removal being essential as any remaining precipitant can occlude light passing through the sample from the plate reader thus resulting in a false positive or excessive result.
Colorimetric analysis
Aliquots (210 µl) of 0.2M acetate buffer pH 5.0 were pipetted into the wells of a 96 well microtitre plate followed by 12 µl of chloramine-T (5g/L in deionised water). 30 µl of neutralised digestion solution was then added followed by 48 µl of isonicotinic/barbituric acid colorimetric reagent prepared by making 0.85 g of isonicotinic acid and 0.90 g of barbituric acid up to 40 ml with deionised water and adjusting the pH to 11.0 with 3.6 M NaOH before making the volume up to 50 ml with deionised water.
The solutions were allowed to develop for 60 minutes before having their absorbance read at 600 nm on a plate reader.
Results
Non-cyanogenic glycoside containing potato chips:
Salt and vinegar potato chips were also assayed by the method above and found to contain < 10 mg/kg cyanide as HCN.
Standard curves
Standard curves were linear across the range 0.5 to 20.0 mg/L as HCN with r2 values ranging from 0.998 to 1.000.
Spiked recovery
The recovery of pure linamarin from a 59 mg/L linamarin solution in 0.1M H3PO4 (equivalent to 6.4 mg/L as HCN) under the conditions described was 96.0%.
The recovery of cyanide as HCN from cassava spiked with linamarin at concentrations from 52-64 mg/kg as HCN ranged from 87-100% (n=5).
Inter-laboratory comparison
As part of the method validation procedure, sub-samples of cassava assayed by the ESR chemistry laboratory were submitted to The Division of Analytical Laboratories, Sydney West Area Health Service, New South Wales, Australia for confirmatory analysis (Table 2).
Table 2. Inter-laboratory result comparison cyanide as HCN in cassava.
Limit of detection (LOD)
The limit of detection for this analysis was < 2 mg/kg total cyanide as hydrogen cyanide.
Discussion
According to the Australia New Zealand Food Standards Code, Standard 1.4.1 Contaminants and Natural Toxicants9 the maximum permitted levels of hydrogen cyanide (mg/kg) in various foodstuffs are: confectionary (25 mg/kg), stone fruit juices (5 mg/kg), marzipan (50 mg/kg) and alcoholic beverages (1 mg per 1% alcohol content).9
In 2008 a limit of 10 mg/kg total cyanide as hydrogen cyanide in cassava chips was set by Food Standards Australia New Zealand (FSANZ) due to concerns about very young children consuming ready to eat cassava chips.10
The method described above is in our opinion sensitive enough and sufficiently reproducible to be applied to the quantitative determination of CNG content in foods for regulatory purposes.
References
1. Simeonova, F.P.; Fishbein, L. World Health Organisation Concise International Chemical Assessment Document 61. Hydrogen cyanide and cyanides human health aspects. 2004
2. Chemwatch Material Safety Data Sheet Chemgold III MSDS database: potassium cyanide http://jr.chemwatch.net/chemgold3/?X (accessed 06/07/2012).
3. Haque, M.R.; Bradbury, J.H. Food Chem. 2002, 77(1), 107-114
4. JECFA (1993) Cyanogenic glycosides in: toxicological evaluation of certain food additives and naturally occuring toxicants. Geneva, World Health Organisation, 39th meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series 30. http://www.inchem.org/documents/jecfa/jecmono/v30je18.htm (accessed 06/07/2012).
5. New Zealand Food safety Authority (NZFSA) Cyanogenic glycosides – Information Sheet http://www.foodsafety.govt.nz/elibrary/industry/Cyanogenic_Glycosides-Toxin_Which.pdf (accessed 06/07/2012).
6. Sang-A-Gad, P.; Guharat, S.; Wananaukul, W. Clin. Toxicol. 2011, 49(9), 834-839.
7. Agency for Toxic Substances & Disease registry (ATSDR) Toxicological Profile for Cyanide Chapter 3 “Health Effects” http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=72&tid=19 (accessed 06/07/2012).
8. Nambisan B. Food Chem. Toxicol. 2011, 49(3):690-693.
9. Australia New Zealand Food Standards Code Standard 1.4.1 Contaminants and Natural Toxicants http://www.comlaw.gov.au/Series/F2008B00618 (accessed 25/07/2012).
10. Food Standards Australia New Zealand (FSANZ) Preparing cassava and bamboo shoots to eat factsheet http://www.foodstandards.gov.au/scienceandeducation/factsheets/factsheets/cassavaandbambooshoo5334.cfm (accessed 25/07/2012).