Winter Zakaria
The NZIC Conference this year was another showcase of quality research being conducted around the country and out in the world. I work with Chris Fitchett at the University of Canterbury. I had the privilege of presenting on a portion of my PhD work that is part of an ongoing project in the group – developing a novel ruthenium-based multi-electron photosensitiser.
The big idea behind the project is mitigating fossil fuel combustion by introducing new methods for fuel production from renewable sources. Photosensitisers of this type have been shown to facilitate the hydrogen evolution reaction using light and extra electrical potential. We have been working on designing a complex that would have a reduced or non-existent electrical potential requirement.
I have been able to synthesise a related complex with different substituents to investigate and compare their physical properties against those previously synthesised. I briefly covered the synthesis of the ligands and complexes that have been produced by the group so far, as well as some preliminary physical characterisation that is looking promising. I feel very proud that my first talk given at a conference was at NZIC. It was a good experience that I have learned from.
The other research and work I was particularly interested in were those related to science communication, chemical education, and outreach. The talks given by Professor Alice Motion (University of New South Wales), and Associate Professor Anna Garden (University of Otago) were especially captivating and inspiring. Throughout these talks there was a theme of innovation – new methods of communication and setting students up to learn more effectively.
Alice Motion gave a plenary lecture on science communication and outreach with the public. She covered various media and events she had played a part in, including radio, stage shows, and fashion showcases. The project that interested me the most was on a completely auditory experience of the model of an atom, through what is termed a ‘sonaphor’, short for sonic metaphor (Weaver, A.; Firmer, G.; Motion, A. et al. Sounding out science: the sonaphor and electronic sound design as a learning tool in secondary science, Postdigit Sci Educ 5, 408–439 (2023), https://doi.org/10.1007/s42438-022-00321-4). Her team designed a soundscape accompanied by English narration to translate what an atom is like through sound only. It was something that had never been brought up before, but atomic theory is usually conveyed by imagery, such as Bohr’s ‘planetary model’. Something clicked when I heard the sonaphor for the first time. Experiencing a concept that I was very familiar with in a way that I had never experienced it before was meaningful for me. Chemists now more than ever are interested in discovering new tools to put in our belts or polishing old tools to work better for us. This project introduces a new tool to convey chemistry with, and I cannot wait to see what else they have to offer.
Anna Garden gave a talk on an electronic interactive lesson course on nanoparticles and computational chemistry which was developed by a former student of hers. The course featured an introduction to nanoparticle formation and stability, and let students try out a bit of computational chemistry for themselves. The tool is intended for first-year students as an introduction to nanoscience. It is an open-source project being hosted on GitHub, so it is open for anyone to use for free following this link and the instructions on the website: https://github.com/GardenGroupUO/Computational_Silver_Nanoparticle_Exercise. It is a nice way to introduce students to computational chemistry, even if it is just a small taste.
I enjoyed my time at the conference, getting caught up on the recent advances in chemistry. While many of the synthetic projects were interesting too, I think I got the most out of the presentations on science communication. It is becoming increasingly important, given the falling trust and enrolments in STEM. It is not enough for the subjects themselves to be interesting or important for career-making, there needs to be interaction and engagement with our audiences. These projects show how that can be achieved with the public and in the classroom.
Nicole Soriano Ladino
The NZIC Conference, held at the University of Otago in November 2024, brought together researchers and industry leaders to present their work in fields such as medicinal chemistry, chemical biology, inorganic and organometallic chemistry, chemical education, and analytical and physical chemistry. This conference provided an excellent opportunity to exchange ideas, showcase research, and foster collaborations.
I was fortunate to be one of the presenters and delivered a 15-minute presentation titled “Synthesis and optimisation of novel anti-TB agents.” This presentation was part of the organic and medicinal chemistry stream, which aligned well with my research. My work focuses on developing a robust synthetic route for producing final compounds tested against Mycobacterium tuberculosis. Presenting at the conference not only allowed me to share my findings but also helped me gain valuable feedback from experts in the field.
The conference featured numerous insightful talks, including several presentations and posters on prodrug synthesis. One particularly engaging talk was delivered by Thomasin Brind titled, “Amplification of biorthogonal ‘click-to-release’ prodrugs.” This presentation focused on the synthesis of thiocarbamates, which play a dual role in drug release and self-propagation, leading to self-amplification. The innovative chemistry behind these compounds has significant potential for controlled drug delivery applications as you can track how much drug is being released at any given point in time.
Another fascinating presentation was given by Jessica Fairhall titled, “Acid-cleavable polymer-drug conjugates for targeted delivery to the stomach.” Although not directly related to my research, it introduced an innovative approach to drug conjugation. Her work explored polymer-drug conjugates that become active in response to stomach pH, providing a promising targeted treatment for chronic stomach diseases. Given the challenges in diagnosing and treating such conditions, this strategy could lead to significant advancements in pharmaceutical chemistry.
A key focus of my interest at the conference was research on tuberculosis (TB) and its pathogen, Mycobacterium tuberculosis. Although TB was mentioned in various talks, only two presentations focused solely on targeting this pathogen, one of them being mine. The second was delivered by Chelsea Tyler titled, “Synthesis and biological evaluation of antitubercular inhibitors.” Her research explored an innovative dual-target inhibition approach, aiming to block two protein targets within the electron transport chain of Mycobacterium tuberculosis. Her findings provided a unique perspective on multi-target drug development and the potential to enhance antitubercular treatment strategies. Additionally, I was surprised to learn that a laboratory in New Zealand is still conducting in vitro studies using Mycobacterium tuberculosis, as we thought that this kind of work had stopped a while ago.
I found the plenary and keynote speakers to be particularly engaging. Their talks provided valuable insights and broader perspectives on chemistry-related advancements, many of which, while not directly linked to my field, were very interesting and inspiring.
Finally, I would like to thank the NZIC Canterbury branch for their financial support. This funding enabled me to focus on my presentation and provided the opportunity to network with researchers from various chemistry disciplines. Attending this conference was a rewarding experience, reinforcing the importance of collaboration and knowledge exchange in advancing scientific research.
Rajika Munasinghe
The NZIC Conference 2024 was an enriching platform to present my research and engage with the broader chemistry community. This conference brought together academics, industry professionals, and students to exchange ideas and showcase advancements in chemistry.
I had the opportunity to present an oral presentation on my research titled, "Approaches to photoactivated cytotoxins," which explores innovative methods for synthesising heterodinuclear systems with potential applications in photoactivated chemotherapy. This experience was both intellectually stimulating and professionally rewarding. My research work focused on the synthesis and study of Zn(II) porphyrin-Co(III) heterodinuclear systems (Fig. 1), aiming to investigate their potential in photoactivated ligand release.
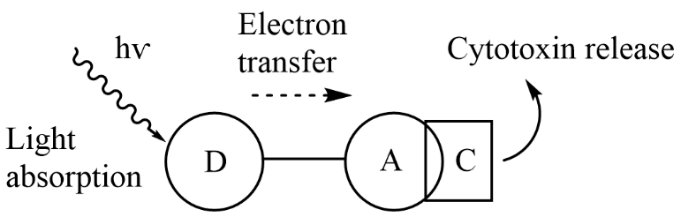
During the talk, I discussed the synthesis of Zn(II) porphyrin-Co(III) heterodinuclear systems using four synthetic stages:
- Synthesising Zn(II) porphyrin-based electron donor component.
- Elaborating this component to build a second metal-binding site using reductive amination and amide coupling reactions.
- Model complexation studies using Co(III)-amine-triflato complexes and demonstrating the photoactivated ligand release of heterodinuclear systems.
- Developing cytotoxic Co(III) nitrogen mustard complexes with labile ligands such as triflate and nitrile.
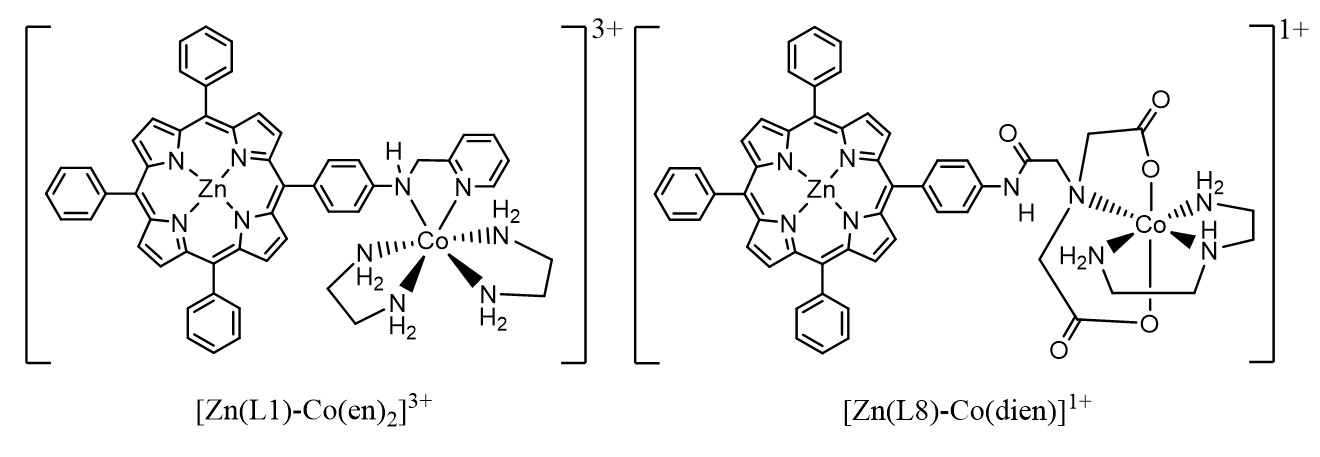
Key findings included synthesising six new Zn(II) porphyrin complexes using reductive amination and amide coupling reactions and two model heterodinuclear systems (Fig. 2) and the efficient photoactivated ligand release of the [Zn(L1)-Co(en)2]3+ system within 30 minutes. This research highlights the potential of light-sensitive prodrug systems for targeted cancer therapy and provides a foundation for future exploration in this area.
Several presentations at the conference resonated with my research interests. One particularly notable keynote talk was by Dr Siddharth Matikonda on a novel approach to cell labelling using cyanine phototruncation. This talk and discussion with Siddharth provided valuable insights on how this concept could be used effectively in targeted cancer therapy.
The industry speaker, Dr Shalini Divya, shared her inspiring journey from being a student to becoming a start-up co-founder and chief executive officer. The panel discussion during the industrial session was also engaging, offering valuable insights into the viewpoints of industry professionals.
Beyond the talks, I appreciated the opportunity to connect with peers, academics, and industry professionals. I also had the privilege of meeting Professor James Crowley, the external examiner for my PhD defence. Our discussion about my thesis and research findings was both memorable and motivating.
The poster session gave me an excellent opportunity to link with peers. It provided insight into the wide range of research currently conducted in chemistry. I enjoyed learning about the findings presented by familiar faces from the University of Canterbury. I had the pleasure of meeting two Sri Lankan researchers, which added a personal touch to the experience.
Overall, the conference expanded my knowledge of chemistry and gave me an understanding of current academic and industrial research trends and practical challenges. Attending the NZIC 2024 Conference has been an invaluable experience, broadening my knowledge of modern research trends and refining my scientific perspective. This experience will undoubtedly influence my future contributions to the field of chemistry.
I sincerely thank the Canterbury Branch of the NZIC for supporting my participation in this conference. Thanks to my research supervisor, Prof. Richard Hartshorn and co-supervisor, Prof. Rudi Marquez-Mazlin, for their guidance and mentorship throughout my research journey and my husband, Chinthaka Tennakoon, family and friends for their continuous support and encouragement.
Askin Eldiven
The NZIC 2024 conference presented a unique opportunity to delve into innovative research areas in chemistry while exchanging ideas with peers and experts. My presented research focused on detecting volatile organic compounds (VOCs) of ionic liquids (ILs) during pyrolysis reactions, showcasing a novel mass spectral technique that addresses current limitations in thermal stability analyses.
Ionic liquids (ILs) have emerged as versatile materials with applications spanning electrochemical systems, solar power, and beyond. However, the operational stability of ILs under extreme conditions is a growing concern. My research addresses this by understanding their decomposition mechanisms during pyrolysis reactions. Unlike traditional methods, which rely on techniques such as TGA-MS, GC-MS, or Pyr-MS that often involve hard fragmentation and overlapping fragments, our novel method provides cleaner, more interpretable data by avoiding hard fragmentation. By monitoring the VOCs released during pyrolysis, we identified specific decomposition pathways and opportunities to control the process. This contributes to the development of more robust ILs, ensuring their durability in practical applications.
The conference featured a range of thought-provoking presentations. I gained valuable insights from the PhD students of Patricia Hunt, who presented innovative work on the characterisation techniques of imidazolium-based ILs and hydrogen halide stabilisation in ILs. Their detailed analysis demonstrated new ways to understand the chemical behaviour of ILs, which could inspire improvements in IL design for diverse applications.
Another highlight was Terry Francombe's presentation, which provided a deeper understanding of detecting oxygen using the O-1s region with an X-ray photoelectron spectroscopy measurement technique and clarified a major misunderstanding in interpreting the spectra. His ability to bridge fundamental concepts with practical implications was truly enlightening and has prompted me to consider not always trusting common interpretations of spectra and being more self-guided in my own analyses in the future.
A personal highlight was reuniting with some PhD students and postdocs I had previously met at a workshop organised by the MacDiarmid Institute’s student body, MESA. It was a joy to reconnect with them, share updates on our respective research journeys, and discuss potential collaborations. These encounters reminded me of the importance of community within the scientific field, where shared experiences and networks can lead to new ideas and partnerships.

Beyond the conference sessions, the city of Dunedin itself added to the experience. The architectural charm and cityscape were fascinating, to say the least, with historic sites and vibrant streetscapes providing a picturesque environment for the event. The culinary diversity offered plenty of opportunities to explore local cuisine, and it was refreshing to combine academic engagement with cultural exploration. This mix of intellectual engagement and cultural immersion made the event even more memorable.
Attending this conference reinforced the importance of interdisciplinary approaches to address complex challenges. The exposure to cutting-edge techniques and diverse viewpoints in different areas of chemistry and materials science has inspired me to take a new approach to the methodology in my research. And moving forward, we aim to explore additional IL designs to further optimize their thermal properties, ensuring broader applicability and resilience.
I am grateful to the Canterbury Branch of the New Zealand Institute of Chemistry for supporting my participation in this event. The insights gained, the connections formed, and the experiences shared have been invaluable in advancing my research and contributing to the broader field of chemistry.
Alexis Blackie
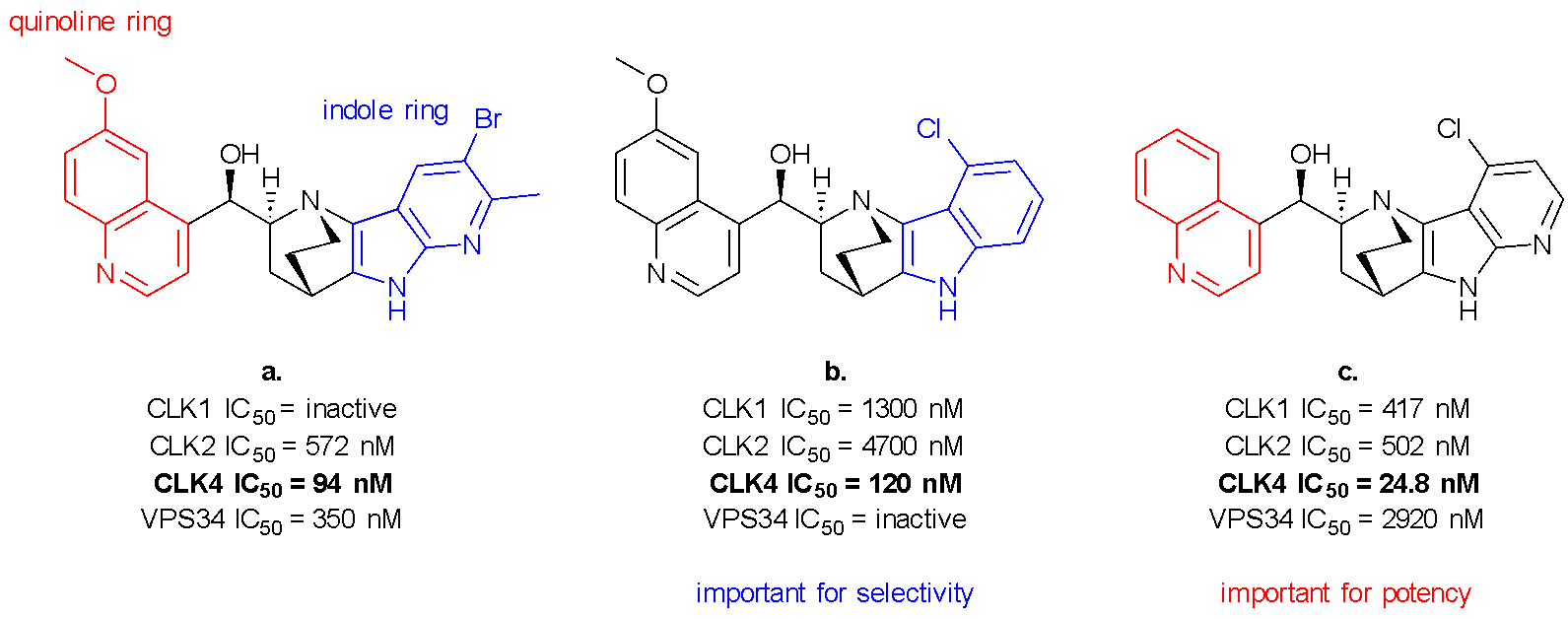
I recently had the opportunity to present a summary of my work at the NZIC Conference 2024 at the University of Otago in Dunedin. I am interested in the Cdc-2-like kinases (CLKs), which are a family of relatively underexplored kinases that have been linked to the spliceosome and its involvement in cancer development and progression.1 CLK4 in particular has been linked to metastatic triple-negative breast cancer (TNBC).2 TNBC is difficult to treat because it lacks the receptors that are usually used to target breast cancer, and is also very prone to developing drug resistance.3 Our lead compound, azaquindole-1 (Fig. 1a), is able to inhibit CLK4 and several other kinases in vitro, but the phenotype of cells treated with azaquinole-1 is currently dominated by the inhibition of starvation- and rapamycin-induced autophagy arising from modulation of lipid kinase VPS34.4 Because of its CLK4 activity, it is likely that the azaquindole scaffold will be suitable for development into a selective inhibitor able to target TNBC and other diseases related to spliceosome malfunction.
Over the course of my PhD, I have refined the original route to improve the yield of each step and simplify where possible, and then applying this new route to the synthesis of novel azaquindoles to continue exploring the scaffold’s SAR. Over several rounds of design and synthesis we have determined that the substitution pattern of the indole ring is important for selectivity (Fig. 1b), while modifications to the quinoline ring can influence potency (Fig. 1c). In the final months of my project we aim to combine these key features to create an inhibitor that is both potent and selective for CLK4.
I also enjoyed attending the talks and getting to hear about different types of research happening around the world. I found those by Professor Jeff Keillor (University of Ottowa, “Targeted covalent inhibition of tissue transglutaminase”) and Associate Professor Jonathan George (University of Adelaide, “Synthetic studies on Australian natural products”) particularly interesting.
The Keillor group works at the interface of chemistry and biochemistry to study the structure, function, and inhibition of enzymes using chemical tools. As someone who also makes inhibitors, I found Jeff’s explanation of the key steps required to turn a selective inhibitor into a drug (confirmation of mechanism of action, cellular distribution, activity in cells and biological models, etc) to be very thought-provoking.
The George group takes inspiration from nature and natural biosynthetic pathways, particularly those that employ unusual rearrangements, to develop concise routes for the total synthesis of natural products. One impressive example shared at the conference showed five stereocentres, four C-C bonds, and four rings formed in one step during the synthesis of garcibractetone.5

I also attended some of the talks outside my normal area of interest (organic chemistry) to see what some of the other groups are working on. Many inorganic chemists at the conference were also working on anti-cancer drugs (Dr Tameryn Stringer, University of Waikato; Professor James Crowley, University of Otago), but they usually have very different modes of action to organic/small molecule therapeutics so it was good to be exposed to some different types of chemistry that encouraged me to think in different ways.
I would like to say thank you very much to the NZIC Canterbury Branch for the supporting me so I could have this experience! 😊
References
- Araki, S.; Dairiki, R.; Nakayama, Y.; Murai, A.; Miyashita, R.; Iwatani, M.; Nomura, T.; Nakanishi, O. Inhibitors of CLK Protein Kinases Suppress Cell Growth and Induce Apoptosis by Modulating Pre-MRNA Splicing. PLoS One 2015, 10 (1), e0116929. https://doi.org/10.1371/journal.pone.0116929.
- Kang, E.; Kim, S.; Jeon, S. Y.; Jung, J. G.; Kim, H. K.; Lee, H. B.; Han, W. Targeting CLK4 Inhibits the Metastasis and Progression of Breast Cancer by Inactivating TGF-β Pathway. Cancer Gene Ther 2022, No. December, 1–13. https://doi.org/10.1038/s41417-021-00419-0.
- Schmadeka, R.; Harmon, B. E.; Singh, M. Triple-Negative Breast Carcinoma Current and Emerging Concepts. Am J Clin Pathol 2014, 141, 462–477. https://doi.org/10.1309/AJCPQN8GZ8SILKGN.
- Foley, D. J.; Zinken, S.; Corkery, D.; Laraia, L.; Pahl, A.; Wu, Y. W.; Waldmann, H. Phenotyping Reveals Targets of a Pseudo-Natural-Product Autophagy Inhibitor. Angewandte Chemie - International Edition 2020, 59 (30), 12470–12476. https://doi.org/10.1002/anie.202000364.
- Pepper, H. P.; Lam, H. C.; Bloch, W. M.; George, J. H. Biomimetic Total Synthesis of (±)-Garcibracteatone. Org Lett 2012, 14 (19), 5162–5164. https://doi.org/10.1021/ol302524q.
Michelle Bailey
The 2024 NZIC Conference brought scientists from across the country to Dunedin for an exchange of new research findings spanning the entire field of chemistry. From natural products to computational chemistry, or drug development to metalorganic frameworks, the conference showcased the diversity of chemistry and its importance in addressing different modern challenges. Personally, I was particularly drawn to the biochemistry-focused presentations, as they best aligned with my background, current research interests and inspiring directions for future work.
The biochemical work presented at the NZIC conference highlighted the importance of this sub-category of chemistry in addressing some of the world's most pressing challenges. For example, Professor Emily Parker’s talk focused on fungal indole diterpenes, a structurally diverse class of natural products with anti-bacterial, anti-viral, and anti-cancer properties. By reconstructing different indole diterpene biosynthetic pathways, her group was able to increase the accessibility to and uncover a range of new promising compounds. Similarly, the work of Professor Jeffery Keillor’s group also highlights the importance of biochemical findings for drug discovery and medicinal chemistry. His talk centred on the development of peptidomimetic-based inhibitors targeting tissue transglutaminase by preventing it from accessing a conformation important for the survival of stem cell cancer cells. Another talk I enjoyed listening to was Tristan de Rond’s, as his group approaches biochemistry from an exciting angle, starting with rare gene discoveries and exploring their functions and potential applications in chemical biology and industrial synthesis.
Attending the conference also offered the opportunity to present some of the work I have carried out over the first nine months of my PhD in form of a poster. My research focuses on a group of enzymes involved in the biosynthesis of polyhydroxyalkanoates (PHAs), which are a type of biopolymers some bacteria can produce under nutrient-limited conditions. This is a way for the bacteria to store reserves of energy, which they can break down for later use. There is a complex array of enzyme machines that make and break down these PHAs in the bacterial cell, with different bacteria having diverse classes of the PHA synthases. As details about the molecular level processes of PHA biosynthesis remain elusive, part of my PhD project focuses on characterising class IV PHA synthase complexes. By recombinantly expressing and purifying both enzyme components of the complexes (PhaC and PhaR), their interactions, structures and activities will be investigated and compared to the better-understood class I PHA synthases. PHAs are interesting materials, due to sharing similar properties to conventional, petroleum-based plastics, while additionally being biodegradable and derived from renewable sources. Through improved understanding of the mechanisms underpinning PHA synthesis, my research aims to aid in the downstream optimisation of industrial PHA production. I received very positive responses to my poster and am grateful to everyone asking engaging questions, offering valuable feedback and providing insightful discussions. Additionally, I am very thankful to the NZIC for having received one of the student poster prizes.
Attending the NZIC conference was a rewarding and inspiring experience. While it was particularly engaging to interact with biochemistry-focused sessions, the conference also offered a fascinating glimpse into other branches of chemistry. Hearing about the advances in synthetic, inorganic and computational chemistry added to my appreciation for how different disciplines within chemistry collectively contribute to problem-solving on a global scale, and the interdisciplinary nature of modern chemistry.
Thanks to being a recipient of the “NZIC Travel Grant” provided by the Canterbury branch, I was able to attend this gathering, which proved to be an amazing opportunity to connect with New Zealand’s scientific community, exchange ideas, and learn from diverse perspectives, as well as present my research. I am very grateful for this opportunity to learn from leading researchers, build connections, and find inspiration for my continued professional growth.
Imogen Alderson
I am a third year PhD student studying at the University of Canterbury under the supervision of Dr Daniel Foley. Recently I had the opportunity to attend the NZIC 2024 conference at the University of Otago and I presented a summary of some of the work I have been doing towards my PhD at the poster session of this conference.
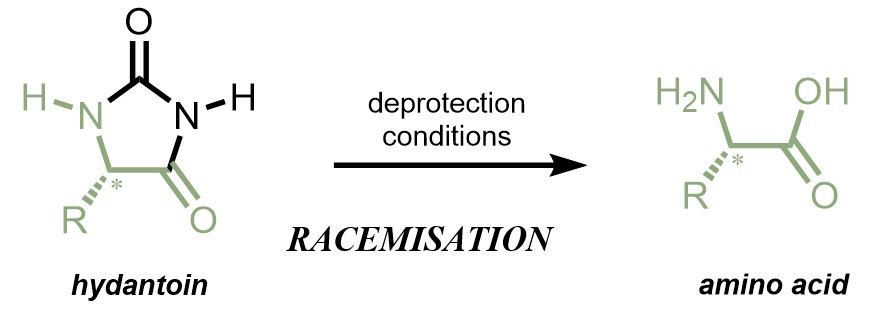
I am interested in the synthesis and deprotection of hydantoins. Hydantoins are nitrogen containing 5-membered rings (Fig. 1) that are a prevalent scaffold in medicinal chemistry - their structure contributes to many drug compounds currently on the market.1 Hydantoins are also known to be synthetic precursors to amino acids. Using various synthetic transformations, the hydantoin can be hydrolysed and the corresponding amino acid can be obtained (Fig. 1).2 Nature completes this process using a cascade of enzymes in the hydantoinase process; this is a 3 step process and is structurally specific.3 However, there are currently no chemical procedures (rather than biochemical) that enable this transformation without racemisation of the 5-position stereocentre.2 Development of a chemical method to deprotect enantiopure hydantoins into enantiopure amino acids would be valuable for drug discovery efforts.
Amino acids contribute to a class of drugs called peptide therapeutics - drugs that incorporate both proteinogenic and novel amino acids into their structures.4 I presented a summary of the methods I have tested so far throughout my PhD in an attempt to achieve racemisation free deprotection of hydantoins. Unfortunately, we have not had success with method development thus far. Despite this, I enjoyed presenting at NZIC 2024 and had the opportunity to discuss my work with many scientists during the poster session. It was insightful, and I had many productive discussions about alternative methods to investigate.
I enjoyed the conference, and it was great to hear about the different kinds of research occurring in New Zealand and other countries around the world. I was especially interested in the keynote speaker Associate Professor Jonathan George, and a talk presented by Brendon Gill during the RSC Sponsored Industry Session.
The latter talk discussed the separation and isolation of the stereoisomers of α-tocopherol by chiral HPLC. In my research I work with chiral molecules, and use chiral HPLC frequently. Hearing about the different methods that were developed for the separation of α-tocopherol, and the ways that the reproducibility of each method were tested was very interesting.
I attended many talks in the organic chemistry and medicinal chemistry breakout sessions. However, I also attended talks that were outside of my research area and listened to several breakout sessions on inorganic and supramolecular chemistry. I found these talks to be very interesting and it was a great to hear about what other scientists are working on. One of the talks I attended (Jessica Fredericksen, Auckland University of Technology) discussed many of the challenges regarding purification of their target compound using column chromatography, as someone using column chromatography frequently, I found this to be very relatable!
I would like to thank the NZIC Canterbury Branch for the travel grant that supported my attendance at this conference. It was a fantastic experience!
- Aryal, S.; Hone, C.A.; Polson, M.I.J.; Foley, D.J. Chem. Sci. 2023, 14 (29), 7905-7912.
- Konnert, L; Lamaty, F.; Martinez, J.; Colacino, E. Chem. Rev. 2017, 117 (23), 13757-13809.
- Clemente-Jimenez, J.M.; Martinex-Rodriquez, S.; Rodriguez-Vico, F.; Heras-Vazquez, F.J.L. Recent Pat. Biotechnol., 2008, 2, 35-46.
- Blaskovich, M.A. J. Med. Chem. 2016, 59 (24), 10807-10836.
Josh Hughes
From receiving the NZIC student travel grant, I was able to travel down to Dunedin and attend NZIC 2024. Although much of my own PhD research was unable to be presented, I was still gladly able to present some recent research completed within the Foley lab. Within the Foley group we are investigating reactions between dicarbonyl compounds and ureas. Previous research in the group, completed by Sushant Aryal, enantioselectively synthesised a wide range of hydantoins from glyoxals and ureas using chiral phosphoric acids as a catalyst. The hydantoin scaffold is seen in many molecules that exhibit a wide range of biological activities, thus efficient pathways to access these molecules in an enantioselective manner is massively beneficial. I presented this research as my PhD is somewhat a continuation of this work as I am also investigating reactions between dicarbonyls and ureas. I also displayed some of the chiral phosphoric acids I had been making in the lab, as I am currently trying to find an effective catalyst for my own PhD research.
I presented this research at the poster session and found that this was a great opportunity to practice communicating novel research. Getting to have in-depth conversations about the research as people walked around was a fun experience. Furthermore, going to see other peoples’ posters was also beneficial as it allowed me to see not only the other research being done, but also how they decide to visually communicate that research. Observing the variety of presentation styles and creative design choices helped me think about how I could improve my own visual communication in future presentations.
Being able to attend this conference was a great opportunity to see some exciting novel chemistry being completed not only in New Zealand but overseas as well. Some of my favourite presentations to see were the total synthesis presentations, observing all the creative and unique synthetic pathways that were utilised to access compounds found in nature was truly inspiring. I also found that NZIC was a great opportunity to expose myself to chemistry that I’m not overly familiar with, whether it was physical chemistry or computational chemistry, which, although I do not have a great grasp of, I was still able to learn some fascinating concepts from other researchers within these disciplines. Being able to travel to Dunedin also allowed me to meet a lot of other postgraduate chemistry students from around the world and discuss the research they are doing along with the problems that they are facing in the lab. This was incredibly valuable as it allowed us to troubleshoot common issues that we were facing in our research, sharing advice and potential solutions.
Overall, this conference has been a great experience. It has given me much-needed experience in sharing my research and networking with others in the scientific community. I look forward to my next opportunity to present, hopefully my own PhD work, as NZIC has provided me with invaluable experience that will be beneficial for my future career.
Jude Kalan
I am writing to express my sincere gratitude to the New Zealand Institute of Chemistry for awarding me a travel grant to attend and present my work at the recent conference in Dunedin. This support made a significant difference in my ability to participate, and I truly appreciate the opportunity. I would also like to take this opportunity to thank you for giving me the honour of representing Canterbury in the Student Talks competition. Presenting to such a large audience was a great experience and exposure for my work.
My talk was focussed on the work of my PhD about how I am trying to make a model of the glycocalyx (layer of oligosaccharides on the cell surface). The oligosaccharide, the biological structure they are attached to, and their spatial relationship to other glycoconjugates all impact binding affinities and selectivity. This results in the glycocalyx being a complex network crucial for cellular communication that needs to be fully understood. As such, a model on a solid substrate would be an incredibly powerful tool for biologists to probe a variety of interactions. I particularly enjoy sharing this work as it relies on the input of biochemists and other sugar chemists to be valuable, so hearing from the intended users on what is useful and what could be improved on was valuable. I was also pleased to reconnect with Dr Allison Daines from Victoria University of Wellington, who has approached me previously at a difference conference and discussed potential collaborations. It was good to update her on my progress.
Attending the conference was incredibly valuable, not only for presenting my research but also for the learning and connections I gained. In particular, I had been facing challenges with my azide reactions, and I was fortunate to meet experienced azide chemists who provided crucial mechanistic insights. Their presentations made me aware of unexpected and novel pathways for azides and triazoles to decompose, all work conducted in Bill Hawkins group at Otago and Dan Furkerts’ group in Auckland. This then allowed me to come back and try and elucidate whether any of these mechanisms are viable in my case. Their guidance has already influenced my approach, and I am eager to apply their suggestions to improve my work further.
Beyond this, engaging with researchers across different fields expanded my understanding and inspired new ideas for my future work. The discussions, presentations, and networking opportunities were all instrumental in my professional development. I was excited to meet and connect with fellow organic chemistry PhDs and celebrate our small wins and commiserate over failed reactions. As we were all at different institutions, we were able to share little tips and tricks we’ve learnt over our time and bring it over to our own research which has already been very helpful. We also managed to exchange contact information so it was good to start developing my network in the field.
Thank you once again for your generosity and for supporting young chemists like me. I look forward to the next NZIC conference eagerly!




