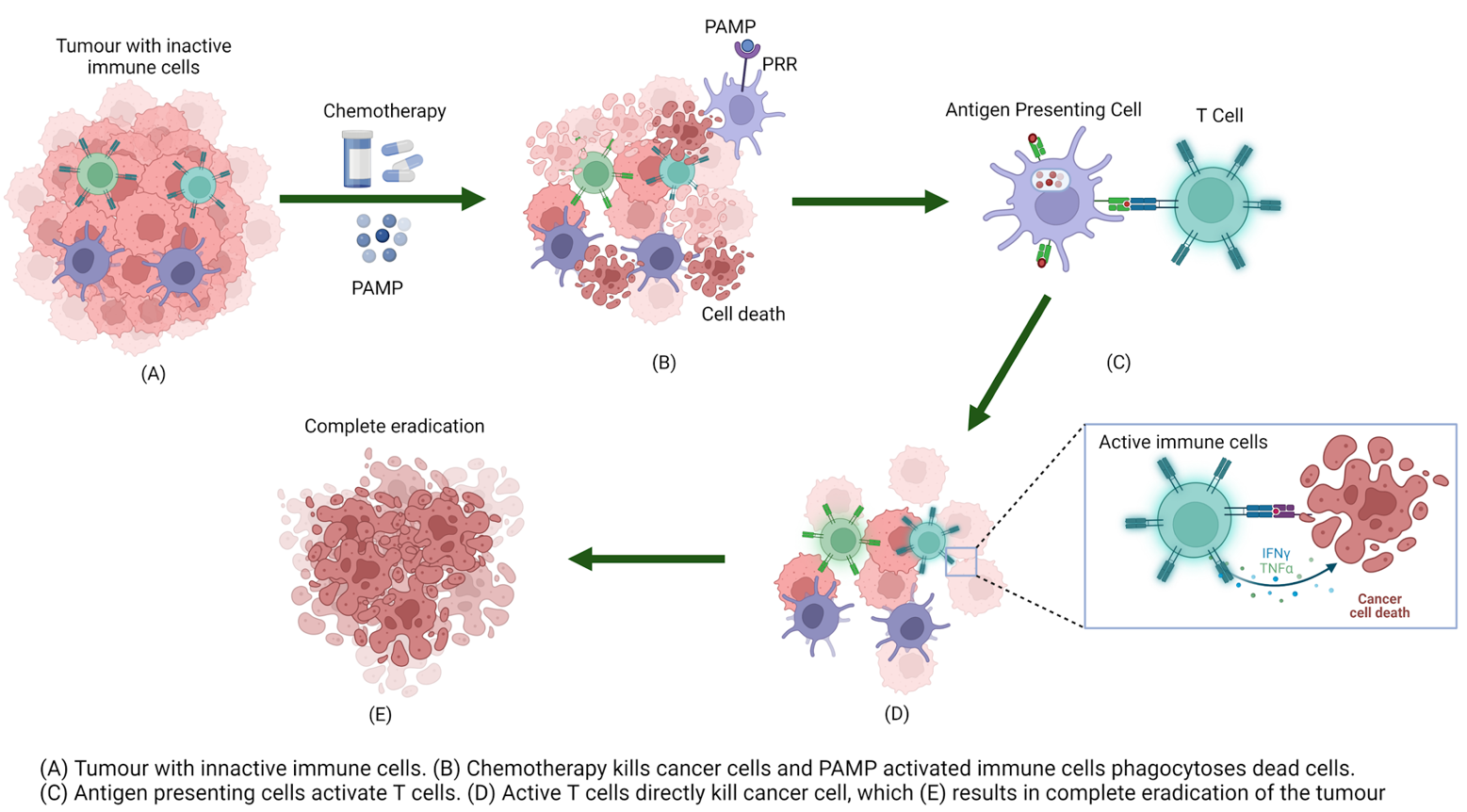
Graphical Abstract
Highlights
- A new cancer treatment option to overcome established cancers
- Synergistic effects seen when combining PAMPs with chemotherapy
- In vitro and in vivo results indicate increased rates of disease-free survival
- Combined PAMP and chemotherapy treatments are now being used clinically
Introduction
Cancer is a severe and often fatal disease affecting millions of people worldwide. In New Zealand in 2018, more than 26,000 people were diagnosed with cancer and over 9,800 people died from the disease.1 The most common cancer treatments are surgery, chemotherapy and radiation.2 For more advanced cancers chemotherapy is the treatment of choice but it is rarely curative for solid tumours.3 Another key drawback of chemotherapy is that it results in severe side-effects.4 Accordingly, studies into alternative methods for the treatment of advanced cancer continue to be developed. The immune system is an important component of the body’s natural defence against foreign infection.5 Immunotherapy is the treatment of disease by either amplifying or reducing the immune response, known as activation or suppression immunotherapy, respectively.6 Amplifying the immune response helps in the host’s detection and destruction of cancer cells, with immunotherapy being considered the newest class of cancer therapy.3 Rational combinations of systemic therapies are often designed to achieve a synergistic anti-tumour response.3 This has led to the investigation of a new concept termed “chemo-immunotherapy”, which is a treatment that combines both immunotherapy and chemotherapy as a novel tactic to overcome established cancers.5,7
Previously, chemotherapy and immunotherapy were considered to be antagonistic.3 This is because chemotherapy kills target cells by apoptosis, which does not result in an inflammatory immune response.3 Another issue is that a common side effect of anticancer drugs is a reduction of lymphocytes which are an important immune cell.3,8,9 However, there is increasing evidence that under some circumstances the combination of immunotherapy and chemotherapy can lead to an anti-cancer immune response greater than either therapy alone, as illustrated in the clinical treatment of various cancers.3,5,10-12 These studies indicate that by activating a long lasting T cell-mediated immune response, the remission and eradication of micrometastases is likely to occur, thus improving chemotherapeutic efficacy.5,13
One way to enhance the immune response is to activate the signalling pathways that respond upon binding of a ligand to a pattern recognition receptor (PRR). PRRs play a key role in the body’s ability to recognise foreign substances, which in turn is an important aspect of cancer treatment. This review will look at current research that combines PRR activation with chemotherapy for cancer treatment.
Pattern Recognition Receptors
Pattern recognition receptors (PRRs) are an important aspect of the innate immune system.14 PRRs recognise pathogen-associated molecular patterns (PAMPs) that are present on microbes such as viruses and bacteria.15 A signalling cascade is instigated upon the binding of a PAMP to a PRR which then leads to the production of cytokines and chemokines and the up-regulation of MHC class II molecules.16 These factors together modulate the immune response. The release of cytokines leads to the development of T cells (Th1, Th17, and CD8+ cytotoxic T lymphocytes cells).17-19 CD8+ T cells are of particular interest as they secrete interferon-γ (IFNγ)- and tumour necrosis factor (TNF)-α which are believed to be able to directly lyse tumour cells.3 This makes PAMP enhanced activation a promising form of cancer treatment.3 Results have indicated that using this treatment in combination with chemotherapy, which also promotes cancer cell death, will result in a synergistic effect in the clinical treatment of cancer.5, 10-12
The classes of PRRs are varied, each receptor is expressed in different cellular compartments and activate specific signalling pathways to generate an immune response.20 This review will focus on the most researched PRR classes that have been used for cancer treatment in combination with chemotherapy, which are the Toll-like, Nod-like, and C-type lectin receptors. This review will analyse the immunostimulatory activities of each PRR class in the context of long-term anti-tumour responses.21 However, it should be noted that there is currently research analysing anti-cancer properties of other PRRs such as RIG-1-like receptors and Cytosolic DNA Sensors, which could lead to future chemo-immunotherapy application.22-25
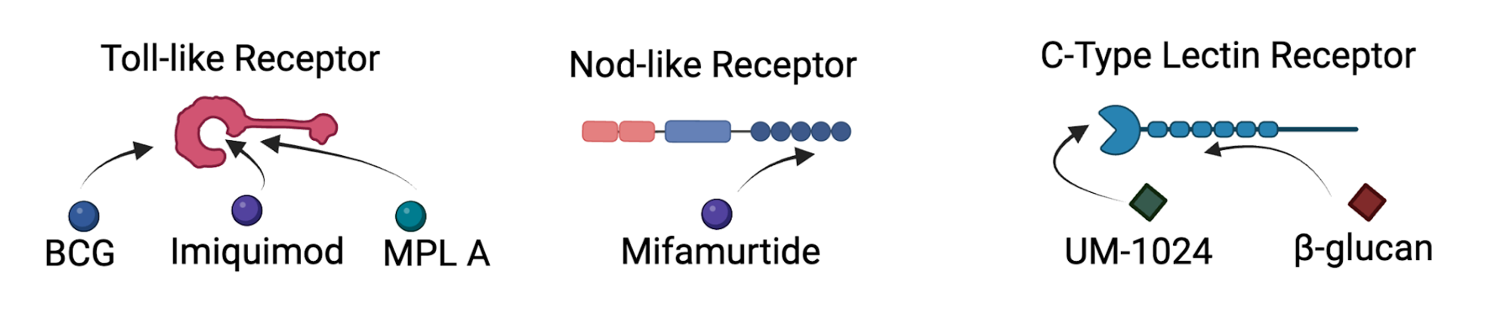
Toll-like receptors
Toll-like receptors (TLR) were the first identified and the best characterised class of PRR.15 TLRs are transmembrane proteins which mean they detect PAMPs either on the cell surface or the lumen of intracellular vesicles such as lysosomes.20 These receptors also detect a range of damage-associated molecular patterns (DAMPs).26 The signalling pathways of TLRs activate the transcription factors NF-κB and AP-1 interferon regulatory factors. This results in the production of cytokines, chemokines and type 1 interferons.15 Research indicates that TLR ligands have anti-tumoral activity against several cancers.21 There are currently three TLR agonists that have been approved by the FDA for use in humans; bacillus Calmette-Guérin (BCG), monophosphoryl lipid A (MPL A) and imiquimod.26 These drugs were originally discovered for their anti-viral and anti-bacterial functions, but further research has indicated their anti-cancer properties.
BCG is currently the gold standard treatment for bladder carcinoma and there has been extensive research investigating its use in chemo-immunotherapy.26 BCG contains ligands that lead to the activation of TLR 2 and 4.27 There are a series of studies that combine BCG and different chemotherapy drugs as treatment for cancer. Each study compared chemotherapy alone and then a combination of chemotherapy and BCG, and while some studies resulted in a marginal or no significant difference when incorporating BCG, in other studies there appeared to be benefit from using the combination therapy. For example, in a study for acute lymphoblastic leukaemia, the effect of chemo-immunotherapy treatment for patients with different prognoses was assessed with BCG augmenting the disease-free survival for certain patient groups.28 A second study looked at this treatment for non-Hodgkin's lymphoma.29 These results indicated that the addition of BCG led to a rate of complete remission of 68%, when compared to chemotherapy alone, which was 48%.29 A promising clinical trial combined BCG with chemotherapy to treat non-muscle invasive bladder cancer, indicating the combination treatment was again effective for certain patient groups.30 Several other studies have illustrated the synergistic effect of BCG and chemotherapy for melanoma, breast, skeletal, lung, colorectal prostate, and gastric cancers, with each study demonstrating an increased rate of disease-free survival for the combined chemo-immunotherapy treatment groups.31-37
Monophosphoryl lipid A (MPL A) is an important component of vaccines against human papillomavirus and hepatitis B virus.21 Use of MPL A results in the rapid activation of an innate immune response.21 This discovery led to further research into the anti-cancer properties of MPL A. MPL A treatment alone is currently in phase II trials for treatment of melanoma and non-small cell lung carcinoma with promising results indicating long-lasting clinical response.21 The chemo-immunotherapy use of this drug has not been studied as extensively, although the combination of MPL A with the chemotherapy drug doxorubicin in the treatment of mammary cancer in mice demonstrates the promise of this approach.38 The results illustrated that the use of MPL A in combination with doxorubicin led to cytokine secretion, which is important for anti-cancer immunity.38,39 A second study involved combing MPL A and mitomycin to treat mice with ovarian tumours.40 The results from this study indicated that the most favourable treatment for the mice was the combination of chemo-immunotherapy.40
Imiquimod is currently used clinically to treat superficial basal cell carcinoma.26 Imiquimod is being investigated in a range of clinical trials for use alone, and in combination with chemotherapy drugs for the treatment of various cancers. Clinical trials have been completed looking at imiquimod with daclizumab/cyclophosphamide and oxaliplatin for brain and colorectal cancer, respectively, although the results from these trails are yet to be released.41,42 An in vitro study treating breast cancer cells with imiquimod and gemcitabine was promising and indicated the use of imiquimod activated immune cells, which in turn helped with tumour suppression.43 An in situ study investigating squamous cell carcinoma also led to promising results for combined therapy, whereby the combination of imiquimod and fluorouracil led to an increased rate of disease-free survival.44
Nod-like receptors
Nod-like receptors (NLR) are a class of intracellular PRRs. This means their key role is to recognise PAMPs that are in the cytoplasm of the cell.15 Two receptors NOD1 and NOD2 sense the presence of peptidoglycan fragments meso-DAP and muramyl dipeptide, respectively.45 When the NLR sense these fragments it drives the activation of transcription factor NF-κB, resulting in the production of cytokines, chemokines, and type 1 interferons.15,45 This immune activation is an important aspect of anti-cancer activity, making NLR ligands an important candidate for chemo-immunotherapy.
There is currently no research investigating cancer treatment using a NOD1 ligand such as meso-DAP. However, there is significant research into muramyl dipeptide analogues as a NOD2 ligand for cancer treatment. Mifamurtide is a synthetic analogue of muramyl dipeptide that has been used to treat lung-resident macrophages, which resulted in the macrophages becoming tumoricidal.46 A study looking at pulmonary metastases in murine models confirmed these results with mifamurtide treatment preventing tumour cell development.46 This led to the investigation of mifamurtide for treatment in human patients where osteosarcoma had recurred with pulmonary metastases.47 In this study patients were treated with a combination of chemotherapy drugs and mifamurtide. When compared to the control group, a statistically significant improvement for patient survival was observed when immunotherapy was incorporated as part of the treatment regime. This led to the clinical approval of this therapy to treat newly diagnosed osteosarcomas.48,49 Further in vitro studies are in progress involving this combination therapy in lung, colon and renal cancer.49 Initial results are promising as treatment resulted in the cytotoxic activation of these cells, giving them tumoricidal activity.
C-type lectin receptors
C-type lectin receptors (CLR) are expressed on the cell surfaces of myeloid cells such as dendritic cells and macrophages. These receptors bind to carbohydrates, often in a calcium dependent manner.50 This preferential binding means that CLRs have a specificity for glycans.51 CLRs are known to regulate a wide range of physiological functions.51 CLRs such as Dectin-1,2 and 3 and Mincle trigger immune responses by helping with dendritic cell maturation, chemotaxis initiation, production of reactive oxygen species and activation of inflammasomes to release cytokines.51 These processes are essential for the development of various T cells, and initiating T-cell based immunity will improve anti-tumour activity.51 This has led to the investigation of CLR ligands in cancer treatment which, though promising, is still in its infancy.17-19, 51
In terms of the chemo-immunotherapy application it is important to note that BCG contains trehalose 6,6′-dimycolate (TDM), a Mincle ligand.52 This means that anti-cancer activity observed in the experiments described previously might also be in part due to the activation of CLRs, though definite studies have not been completed. A study investigating colon cancer in mice combined UM-1024, a Mincle agonist, with the chemotherapy drug cyclophosphamide and showed promising results.52 When UM-1024 was administered alone there was no tumour reduction but when combined with chemotherapy the treatment led to 50% of mice having complete eradication of the tumour.53 The CLR ligand β-glucan is known to play an important role in converting immunosuppressive tumor-associated macrophages (TAM) via the CLR dectin-1.54 TAM have been linked to immunosuppression and resistance to chemotherapy in cancer, making it an important target to investigate further for chemo-immunotherapy.54
Conclusions
It is important to continue to investigate alternative treatments for cancer that will improve recovery and reduce severe side effects. Using chemo-immunotherapy to manipulate the natural immune system and improve the body’s response to cancer treatment has great potential in this field of research. Extensive research has been undertaken analysing a wide range of TLR ligands for use in chemo-immunotherapy treatment. This is presumably due to the early identification of the TLRs and the wealth of knowledge as it relates to ligands and mechanisms that lead to TLR activation.15
The results from the experiments detailed previously illustrate the positive synergistic effects that can be observed when combining TLR ligands and chemotherapy, including a large number of patients experiencing complete remission in some studies.29 Further research should be undertaken for the use of mifamurtide with chemotherapies to treat other cancers, as it is highly effective for osteosarcomas.48,49 In addition, this review highlights a current gap in chemo-immunotherapy research in the other subfamilies of PRRs. In particular, there is limited research on CLRs but initial results using Mincle and β-glucan are promising.53,54 In my opinion future research should investigate how the anti-tumoral properties of NLR and CLR ligands are impacted when combined with chemotherapy to treat cancer cells in vitro.
Acknowledgements
The author would like to thank Dr Emma M. Dangerfield and Associate Professor Bridget L. Stocker for their guidance and feedback during the writing of this article.
All figures were created with BioRender.com
References and notes
- Ministry of Health Cancer data and stats. https://www.health.govt.nz/nz-health-statistics/health-statistics-and-data-sets/cancer-data-and-stats.
- National Library of Medicine Cancer treatments. https://medlineplus.gov/ency/patientinstructions/000901.htm.
- Lake, R. A.; Robinson, B. W. S. Nature Reviews Cancer 2005, 5 (5), 397-405.
- American Cancer Society Chemotherapy Side Effects. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/chemotherapy-side-effects.html.
- Luo, Q.; Zhang, L.; Luo, C.; Jiang, M. Cancer Letters 2019, 454, 191-203.
- National Cancer Institute Immunotherapy to Treat Cancer. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy.
- Wages, N. A.; Chiuzan, C.; Panageas, K. S. Journal for ImmunoTherapy of Cancer 2018, 6 (1), 81.
- Silva, J. What are healthy lymphocytes levels, and what is their function? https://www.medicalnewstoday.com/articles/320987.
- Ménétrier-Caux, C.; Ray-Coquard, I.; Blay, J.-Y.; Caux, C. Journal for ImmunoTherapy of Cancer 2019, 7 (1), 85.
Raj, D.; Agrawal, P.; Gaitsch, H.; Wicks, E.; Tyler, B.Expert Opinion on Pharmacotherapy2021,22(15), 2019-2031- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M. J.; Powell, S. F.; Cheng, S. Y.; Bischoff, H. G.; Peled, N.; Grossi, F.; Jennens, R. R.; Reck, M.; Hui, R.; Garon, E. B.; Boyer, M.; Rubio-Viqueira, B.; Novello, S.; Kurata, T.; Gray, J. E.; Vida, J.; Wei, Z.; Yang, J.; Raftopoulos, H.; Pietanza, M. C.; Garassino, M. C. The New England Journal of Medicine 2018, 378 (22), 2078-2092.
- Cook, A. M.; Lesterhuis, W. J.; Nowak, A. K.; Lake, R. A. Current Opinion in Immunology 2016, 39, 23-29.
- Zitvogel, L.; Galluzzi, L.; Smyth, Mark J.; Kroemer, G. Immunity 2013,39 (1), 74-88.
- Kato, J.; Svensson, C. I., Chapter Nine - Role of Extracellular Damage-Associated Molecular Pattern Molecules (DAMPs) as Mediators of Persistent Pain. In Progress in Molecular Biology and Translational Science, Price, T. J.; Dussor, G., Eds. Academic Press: 2015; Vol. 131, pp 251-279.
- Invivo Gen Innate Immunity. https://www.invivogen.com/review-innate-immunity.
- Yan, H.; Kamiya, T.; Suabjakyong, P.; Tsuji, N. M. Frontiers in Immunology 2015, 6.
- Desel, C.; Werninghaus, K.; Ritter, M.; Jozefowski, K.; Wenzel, J.; Russkamp, N.; Schleicher, U.; Christensen, D.; Wirtz, S.; Kirschning, C.; Agger, E. M.; da Costa, C. P.; Lang, R. PLOS ONE 2013, 8 (1), 53531.
- LeibundGut-Landmann, S.; Osorio, F.; Brown, G. D.; Reis e Sousa, C. Blood 2008, 112 (13), 4971-4980.
- Saijo, S.; Ikeda, S.; Yamabe, K.; Kakuta, S.; Ishigame, H.; Akitsu, A.; Fujikado, N.; Kusaka, T.; Kubo, S.; Chung, S. H.; Komatsu, R.; Miura, N.; Adachi, Y.; Ohno, N.; Shibuya, K.; Yamamoto, N.; Kawakami, K.; Yamasaki, S.; Saito, T.; Akira, S.; Iwakura, Y. Immunity 2010, 32 (5), 681-91.
- Kawai, T.; Akira, S. International Immunology 2009, 21 (4), 317-37.
- Goutagny, N.; Estornes, Y.; Hasan, U.; Lebecque, S.; Caux, C. Targeted Oncology 2012, 7 (1), 29-54.
- Wang, R. F.; Miyahara, Y.; Wang, H. Y. Oncogene 2008, 27 (2), 181-189.
- Goodwin, T. J.; Huang, L. Vaccine 2017, 35 (19), 2550-2557.
- Baba, T.; Yoshida, T.; Tanabe, Y.; Nishimura, T.; Morishita, S.; Gotoh, N.; Hirao, A.; Hanayama, R.; Mukaida, N. Cell Death & Disease 2021, 12 (4), 322.
- Fyrstenberg Laursen, M.; Kofod-Olsen, E.; Agger, R. Cancer Immunology, Immunotherapy 2019, 68 (11), 1875-1880.
- Vacchelli, E.; Galluzzi, L.; Eggermont, A.; Fridman, W. H.; Galon, J.; Sautès-Fridman, C.; Tartour, E.; Zitvogel, L.; Kroemer, G. Oncoimmunology 2012, 1 (6), 894-907.
- Bisiaux, A.; Boussier, J.; Duffy, D.; Quintana-Murci, L.; Fontes, M.; Albert, M. L.; , T. M. I. C. Frontiers in Immunology 2017, 8.
- Odom, L. F.; Tubergen, D. G.; Githens, J. H.; Heideman, R. L.; Blake, M. A. Medical and Pediatric Oncology 1983, 11 (2), 79-90.
- Jones, S. E.; Grozea, P. N.; Metz, E. N.; Haut, A.; Stephens, R. L.; Morrison, F. S.; Talley, R.; Butler, J. J.; Byrne, G. E., Jr.; Hartsock, R.; Dixon, D.; Salmon, S. E. Cancer 1983, 51 (6), 1083-90.
- Huang, D.; Jin, Y. H.; Weng, H.; Huang, Q.; Zeng, X. T.; Wang, X. H. Frontiers in Oncology 2019,9, 121.
- Wood, W. C.; Cosimi, A. B.; Carey, R. W.; Kaufman, S. D. Surgery 1978, 83 (6), 677-81.
- Ochiai, T.; Sato, H.; Hayashi, R.; Asano, T.; Sato, H.; Yamamura, Y. Cancer Immunology, Immunotherapy 1983, 14 (3), 167-71.
- Hortobagyi, G. N.; Yap, H. Y.; Blumenschein, G. R.; Gutterman, J. U.; Buzdar, A. U.; Tashima, C. K.; Hersh, E. M. Cancer Treatment Reports 1978, 62 (11), 1685-92.
- Townsend, C. M., Jr.; Eilber, F. R.; Morton, D. L. JAMA 1976, 236 (19), 2187-9.
- Bjornsson, S.; Takita, H.; Kuberka, N.; Preisler, H.; Catane, H.; Higby, D.; Henderson, E. Cancer Treatment Reports 1978, 62 (4), 505-10.
- Mavligit, G. M.; Gutterman, J. U.; Burgess, M. A.; Khankhanian, N.; Seibert, G. B.; Speer, J. F.; Reed, R. C.; Jubert, A. V.; Martin, R. C.; McBride, C. M.; Copeland, E. M.; Gehan, E. A.; Hersh, E. M. Cancer 1975, 36 (6 Suppl), 2421-7.
- Guinan, P. D.; John, T.; Baumgartner, G.; Sundar, B.; Ablin, R. J. American Journal of Clinical Oncology 1982, 5 (1), 65-8.
- Meraz, I. M.; Hearnden, C. H.; Liu, X.; Yang, M.; Williams, L.; Savage, D. J.; Gu, J.; Rhudy, J. R.; Yokoi, K.; Lavelle, E. C.; Serda, R. E. PLOS ONE 2014, 9 (4), e94703.
- Veglia, F.; Gabrilovich, D. I. Current Opinion in Immunology 2017, 45, 43-51.
- Goldberg, E. P.; Hadba, A. R.; Almond, B. A.; Marotta, J. S. Journal of Pharmacy and Pharmacology 2002, 54 (2), 159-180.
- Immatics Biotechnologies GmbH MA910 Plus GM-CSF With Low-dose Cyclophosphamide Pre-treatment in Advanced Colorectal Carcinoma Patients Following a Successful 12 Week First-line Treatment With Oxaliplatin-based Chemotherapy (IMA910-101) (IMA910-101). https://clinicaltrials.gov/ct2/show/NCT00785122.
- Archer, G. Basiliximab in Treating Patients With Newly Diagnosed Glioblastoma Multiforme Undergoing Targeted Immunotherapy and Temozolomide-Caused Lymphopenia (REGULATe). https://clinicaltrials.gov/ct2/show/NCT00626483.
- Singh, B.; Maharjan, S.; Pan, D. C.; Zhao, Z.; Gao, Y.; Zhang, Y. S.; Mitragotri, S. Biomaterials 2022, 280, 121302.
- Smith, K. J.; Germain, M.; Skelton, H. Dermatologic Surgery 2001, 27 (6), 561-564.
- Kanneganti, T.-D.; Lamkanfi, M.; Núñez, G. Immunity 2007, 27 (4), 549-559.
- Meyers, P. A.; Chou, A. J., Muramyl Tripeptide-Phosphatidyl Ethanolamine Encapsulated in Liposomes (L-MTP-PE) in the Treatment of Osteosarcoma. In Current Advances in Osteosarcoma, Kleinerman, M. D. E. S., Ed. Springer International Publishing: Cham, 2014; pp 307-321.
- 47. Kleinerman, E. S.; Gano, J. B.; Johnston, D. A.; Benjamin, R. S.; Jaffe, N. American Journal of Clinical Oncology 1995, 18 (2), 93-9.
- 48. Meyers, P. A. Expert Review of Anticancer Therapy 2009, 9 (8), 1035-1049.
- 49. Frampton, J. E., Mifamurtide. Pediatric Drugs 2010, 12 (3), 141-153.
- 50. Zelensky, A. N.; Gready, J. E. The FEBS Journal 2005, 272 (24), 6179-6217.
- 51. Brown, G. D.; Willment, J. A.; Whitehead, L. Nature Reviews Immunology 2018, 18 (6), 374-389.
- 52. Hayashi, D.; Takii, T.; Fujiwara, N.; Fujita, Y.; Yano, I.; Yamamoto, S.; Kondo, M.; Yasuda, E.; Inagaki, E.; Kanai, K.; Fujiwara, A.; Kawarazaki, A.; Chiba, T.; Onozaki, K. FEMS Immunology & Medical Microbiology 2009, 56 (2), 116-128.
- 53. Smith, A. Investigation of combinatorial innate immunotherapy with chemotherapy to enhance responses in colon cancer; University of Montana: University Grant Program Reports, 2018.
- 54. Liu, M.; Luo, F.; Ding, C.; Albeituni, S.; Hu, X.; Ma, Y.; Cai, Y.; McNally, L.; Sanders, M. A.; Jain, D.; Kloecker, G.; Bousamra, M.; Zhang, H.-g.; Higashi, R. M.; Lane, A. N.; Fan, T. W.-M.; Yan, J. The Journal of Immunology 2015,195 (10), 5055-5065.

